“The Future is Now” in New Skin Cancer Diagnosis Technology
July 15, 2020
Image
This story has been adapted from the original, posted on the Health Sciences Connect website. A brief video is also available.
The sun’s harmful ultraviolet rays pose elevated risks for skin cancer in the desert southwest, but University of Arizona Health Sciences researchers at the UArizona Cancer Center are using their unique position to address this disease with new technology.
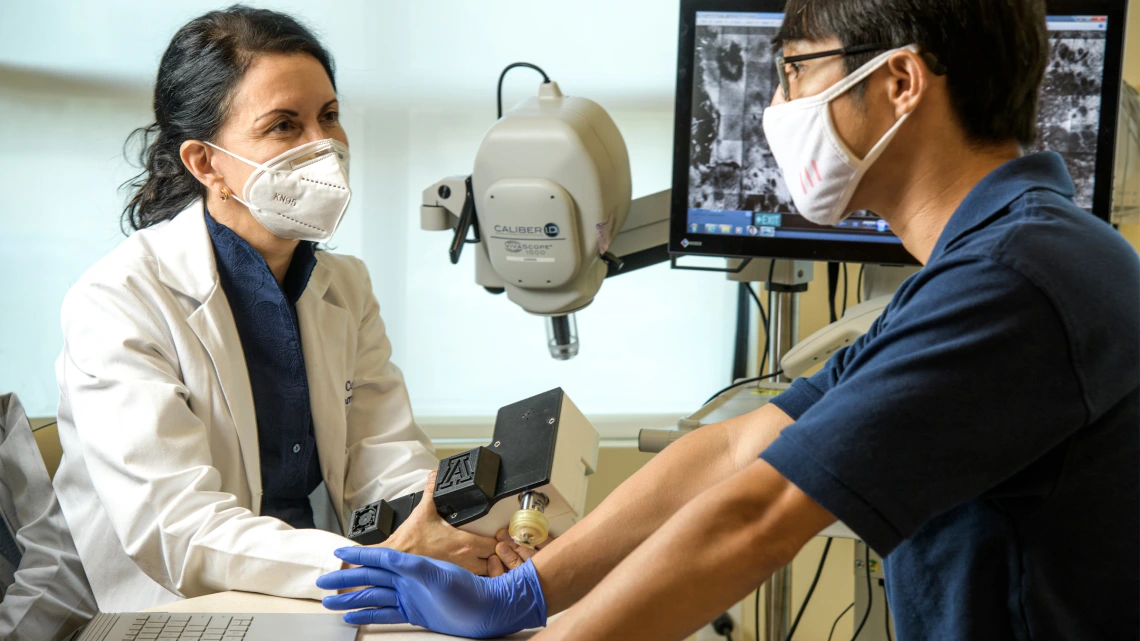
The Center’s Skin Cancer Institute(link is external) has a novel device that provides noninvasive imaging of the skin called reflectance confocal microscopy (RCM). It allows doctors to quickly and safely diagnose many skin cancers and monitor responses to treatment without a biopsy.
Clara Curiel-Lewandrowski(link is external), MD, co-director of the Skin Cancer Institute and interim dermatology division chief at the College of Medicine – Tucson, brought the technology to the university. She is now working with Dongkyun Kang, PhD, co-leader of the Cancer Imaging Program, to create a version of the device that is more accessible to doctors trained in skin cancer diagnosis.
The technology is made with very sophisticated electrical and optical components, according to Dr. Kang, an assistant professor of optical sciences in the James C. Wyant College of Optical Sciences and assistant professor of biomedical engineering in the College of Engineering. His lab has been focused on developing a portable confocal microscope (PCM) using an inexpensive near-infrared LED to lower the manufacturing costs and make the device more practical for clinical use.
Last year, through Tech Launch Arizona, Dr. Kang’s lab licensed three inventions to a startup company in California, which may seek development of a commercial iteration of Dr. Kang’s device. A portable device could look something like a large pen and cost closer to $1,000. Dr. Kang has also tested a smartphone PCM device at the Infectious Diseases Institute (IDI) in Uganda.
“The goal is to make it widely available not just to dermatologists, but to practitioners around the world.” Dr. Kang said. “Collaboration is very important so that we are making this technology work in the context of the clinical setting. I work very closely with Clara and also my collaborators with the project in Uganda to see what we can improve.”
Drs. Curiel and Kang, both members of the BIO5 Institute, continue to advance the PCM device, routinely testing and comparing its performance to the commercial RCM unit and other technologies available.

